MALDI-TOF Mass Spectrometry in the Microbiology Lab
Fast, accurate microorganism identification
Estimated Read Time: 1.5 minutesProtecting Patients Through Fast Identification
Understanding MALDI-TOF Mass Spectrometry
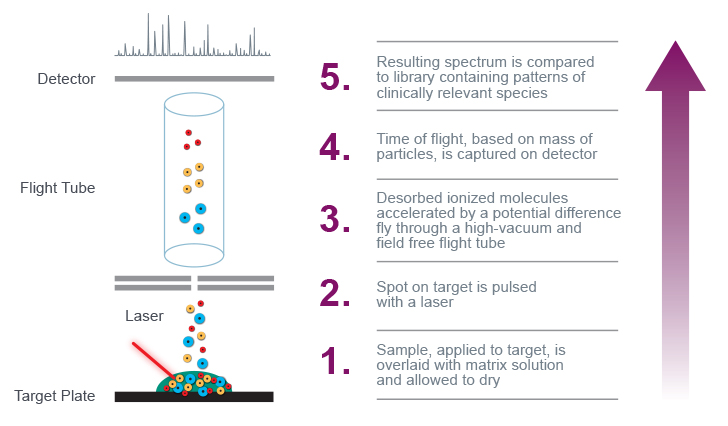
Figure 1. Depiction of internal MALDI-TOF mass spectrometry process.
In mass spectrometry analysis, sample protein molecules are converted into ions in the gas phase and measured for their mass-to-charge (m/z) ratio. A laser strikes the target—a sample fixed to a solid surface with a matrix compound—converting the protein molecules into gas without fragmenting or decomposing them.8
The ionized molecules are subsequently accelerated by a potential difference and fly through the field-free, high-vacuum flight tube towards the detector. The system measures the analytes’ time of flight to the detector and produces a characteristic spectrum. Lighter ions take less time to travel to the detector; heavier ions take more time.
A software application identifies the sample by comparing the resulting pattern of mass peaks to a library of known patterns.
The accuracy of the Bruker MALDI Biotyper as a microorganism identification system, together with its associated libraries, is considered comparable to 16S rRNA gene sequencing (the gold standard) and is globally recognized as the clinical laboratory standard for microbial identification.
Fast, Accurate ID/AST Testing
The DxM Trio delivers rapid mass spectrometry identification, accurate AST results and comprehensive reporting from a single platform. Find out how you can drive advanced levels of efficiency with the combination of the Bruker MALDI Biotyper, the DxM MicroScan WalkAway system and LabPro-MBT software.
2. U.S. Department of Health and Human Services Centers for Disease Control and Prevention. "Detect and Protect Against Antibiotic Resistance." https://www.cdc.gov/drugresistance/pdf/AR_Initiative_Fact_Sheet.pdf. Accessed 23 May 2018.
3. World Health Organization, Europe. "Antimicrobial Resistance." http://www.euro.who.int/en/health-topics/disease-prevention/antimicrobial-resistance. Accessed 23 May 2018.
4. O'Neill, J. "Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations." The Review on Antimicrobial Resistance, 2014. https://amr-review.org/sites/default/files/AMR Review Paper - Tackling a crisis for the health and wealth of nations_1.pdf. Accessed 23 May 2018.
5. Emonet S. et. al. “Application and Use of Various Mass Spectrometry Methods in Clinical Microbiology.” Clin Microbiol Infect. 2010, 16:1604–13.
6. Huang et. al. “Impact of Rapid Organism Identification via MALDI-TOF Combined with Antimicrobial Stewardship Team Intervention in Adult Patients with Bacteremia and Candidemia.” Clin Infect Dis, 2013, 57:1237–45.
7. Perez et. al. “Integrating Rapid Pathogen Identification and Antimicrobial Stewardship Significantly Decreases Hospital Costs.” Arch Lab Med, 2013, 137:1247–54.
8. Creative Proteomics. MALDI-TOF Mass Spectrometry. 2016. https://www.creative-proteomics.com/technology/maldi-tof-mass-spectrometry.htm. Accessed 8 May 2019.
MALDI Biotyper is the property of Bruker Daltonik GmbH.
 English
English


