Clinical Decision-Making Tools for Men’s Health Concerns
Early detection of men’s health concerns through screening tests can lead to better outcomes. This holds true for prostate cancer, which affects approximately 11.6% of men during their lifetime. When the stakes are high, clinicians and patients need tools that can support informed, joint clinical decision making.
Prostate Cancer: to Biopsy or Monitor?
Prostate Cancer Detection Facts
Leading Cause of Cancer Death for Men Worldwide
Men Affected by Prostate Biopsy Complications
Prostate biopsies bear lots of risks, e.g. infection, erectile dysfunction and more. These complications can have a significant impact on a patient’s quality of life.
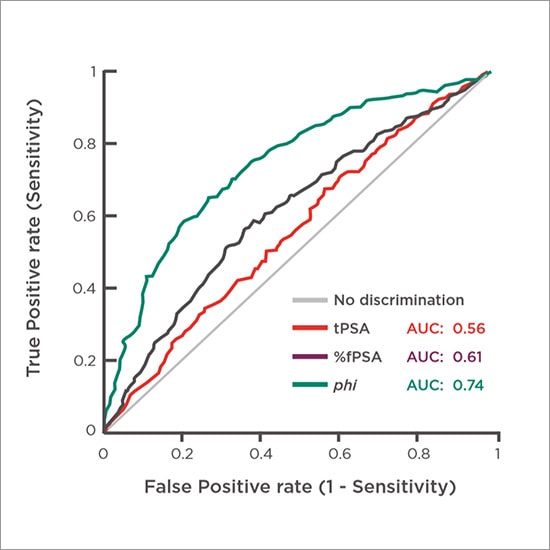
phi is Significantly More Specific Than Other Prostate Cancer Tests
Because phi improves specificity for prostate cancer, it fills the diagnostic gap between PSA screening and prostate biopsy for men whose total PSA falls within the gray zone. Combined with family and patient history, phi can help determine the best individualized patient-management decisions.
Figure 1. Comparison of specificities of total PSA, %fPSA and phi5
Better Prostate Cancer Probability Assessment Than PSA Alone
The superior performance of phi compared to PSA alone has been demonstrated through multiple peer-reviewed publications.
phi in the Real World
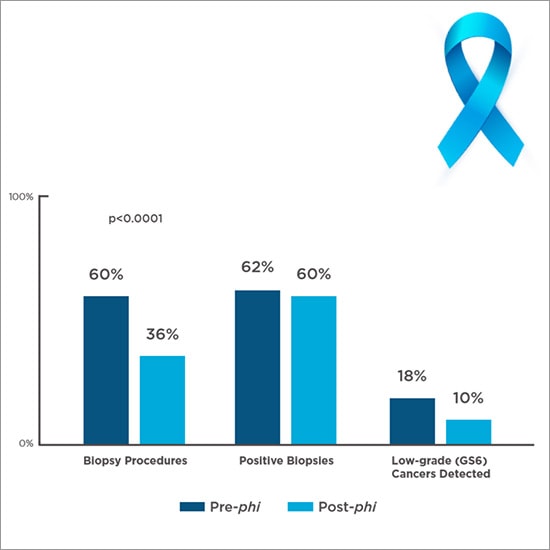
It reduces biopsies when introduced in routine clinical practice. A clinical study (Figure 3) that compared the treatment course of over 500 men assessed with phi to a historical control group—seen by the same urologists within the previous 24 months—showed:
Figure 2. Real-world biopsies and pathological findings before and after phi6
References
1. Hoffman, RM. “Screening for Prostate Cancer.” Updated 16 Jul. 2019. https://www.uptodate.com/contents/screening-for-prostate-cancer . Accessed 14 Aug. 2019.
2. Altok M, Chapin B, Pisters L et. al. “PD43-09 Cost Analysis of Different Prostate Biopsy Modalities.” J Urol, vol. 197, no. 4S, 14 May 2017, p. e821. https://www.auajournals.org/doi/10.1016/j.juro.2017.02.1914. Accessed 1 April 2017.
3. Draisma G, Etzioni R, Tsodikov A et. al. “Lead Time and Overdiagnosis in Prostate-specific Antigen Screening, Importance of Methods and Context.” J Natl Cancer Inst, vol. 1101, no. 6, 2009, pp. 374–383.
1. EAU guidelines: Mottet N, van den Bergh RCN, Briers E, et al. EAU-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer. 2018.
4. U.S. Census Bureau. “American Community Survey 1-year estimates.” Retrieved from “Census Reporter Profile page for United States.” 2017. http://censusreporter.org/profiles/01000us-united-states/.
Accessed 9 Sept. 2019.
5. Stephan et al. “Multicenter evaluation of [-2] proprostate-specific antigen and the prostate health index for detecting prostate cancer.”
6. White J, Shenoy BV, Tutrone RF, et al. “Clinical utility of the Prostate Health Index (phi) for biopsy decision management in a large group urology practice setting.” Prostate Cancer Prostatic
Dis. 2018, 21(1):78–84.
7. Tosoian JJ, Druskin SC, Andreas D, et al. “Use of the Prostate Health Index for detection of prostate cancer: results from a large academic practice.” Prostate Cancer Prostatic Dis. 2017, 20(2):228–233.
8. Catalona W. et al. “A Multi-Center Study of [−2]Pro-Prostate-Specific Antigen (PSA) in Combination with PSA and Free PSA for Prostate Cancer Detection in the 2.0 to 10.0 ng/mL PSA Range” J Urol. 2011 May ; 185(5): 1650–1655. 6. Loeb, S. et al. “The Prostate Health Index Selectively Identifies Clinically Significant Prostate Cancer”. J Urol. 2015;193:1163-69.
phi results are intended to be used as an aid in distinguishing prostate cancer from benign prostatic conditions in men 50 years of age and older with total PSA results in the 2–10 ng/mL range and negative digital rectal examination (DRE) findings. Biopsy outcomes of patients in the 2–10 ng/mL range.
 English
English


