The United States needs up to 193 million tests per month to mitigate COVID-19 to enable the public to safely resume pre-pandemic activities, according to a report issued by the Rockefeller Foundation and Duke-Margolis Center for Health Policy1. Current testing capacity is ~21 million tests per month according to the COVID Tracking Project2. This means that expanded mass testing availability is going to be key to help reopen societies with any level of public confidence.
Challenges in the current diagnostic landscape
PCR is the gold standard for COVID-19 diagnostic testing and is considered to be 98% accurate; but in practice, has not been returning results quickly enough due to testing backlogs at impacted facilities. In addition, the demand of PCR tests does not meet the availability—which increases the severity of the problem. In one analysis, investigators reported that weekly screenings with a variety of less sensitive tests could avoid outbreaks by delivering immediate results and having positive individuals self-isolate3.
Is antigen testing the answer?
Initially, scientists underestimated the benefits of large-scale antigen testing because of their concerns about accuracy. There is a growing belief that fast and accurate antigen tests may help bring the pandemic under control ahead of a possible vaccine4.
With COVID-19 cases surging in Europe, governments in Italy, Spain, Austria and the UK amongst others are turning to new testing methods.
The reason for this is simple. As Professor Christian Drosten, a German virologist who developed the first WHO-approved PCR COVID-19 test said, “What good is a PCR that is very sensitive, but will only deliver results in three, four days because the labs are overburdened?5”
The case for mass testing
For antigen testing to keep the pandemic at bay, vast numbers of the test must be implemented so physicians can spot those who are at the greatest risk of spreading the disease.
Depending upon the antigen test, their sensitivity could be comparable to RT-PCR test sensitivity, enabling real-time results for real-time decision making. However, antigen tests positive results do not always rule out bacterial infections or viral co-infections and negative results do not rule out SARS-COV-2 infection and must be viewed in context with clinical presentation.
According to David Grenache, the chief scientific officer at TriCore Reference Laboratories, lab antigen tests will be much easier to scale up6 and with automated lab systems there may be potentially fewer pre- and post-analytical errors.
How many tests per hour is enough?
Lab tests have a higher throughput, and with the Access SARS-CoV-2 Antigen assay, you can run between 50-200 tests per hour—depending upon the analyzer you use in your laboratory. Because the system is automated, the lab technician has fewer manual steps to follow, thus reducing potential for pre-, post- and analytical errors.
For example, with a point-of-care lateral flow antigen test, one lab technician can run up to 20 tests per hour, which is not particularly conducive to mass testing situations as this will create more—not less— waiting time.
Automated Workflow and Flexible Sample Stability Enable High Volume SARS-CoV-2 Antigen Testing
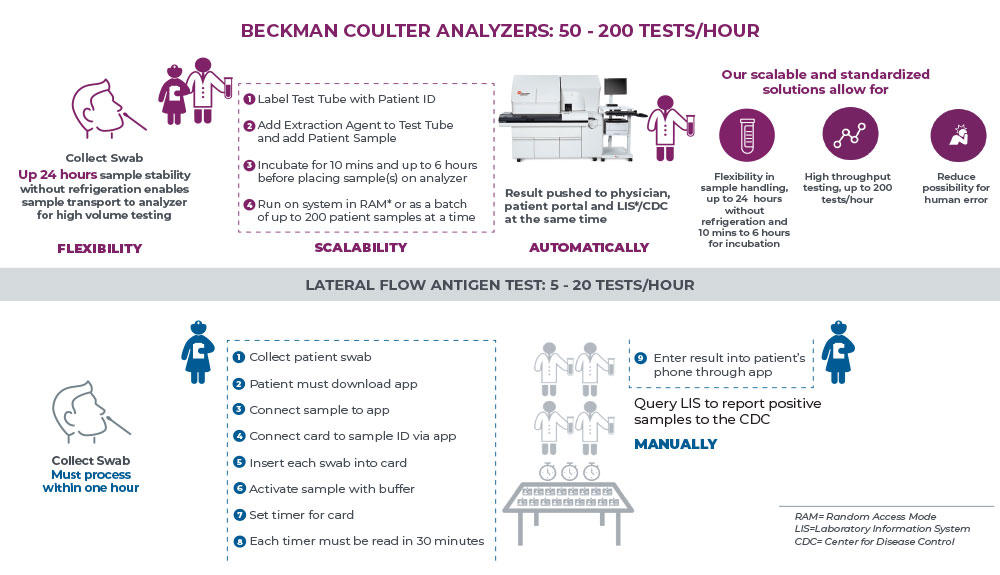
Beckman Coulter’s Access SARS-CoV-2 Ag assay will be produced at mass scale to greatly expand access to testing for people who need it.

 English
English