Why Test Body Fluids?
Body fluid analysis is a challenging task for most labs because the samples—such as cerebrospinal, synovial, pleural fluid, pericardial, peritoneal and general serous fluid are difficult to draw-- not to mention a discomforting experience for the patient.
However, it’s an imperative part of diagnosis, as fluid analysis assists in the identification of certain disease states including meningitis, hemorrhage, malignancy, inflammation, viral, bacterial/parasitic infections and more. Because urgent diagnostic data is often sought after from even a very limited sample for the diagnosis of these disease states, accuracy and speed of results take on even greater importance.
For this reason, laboratories have traditionally resorted to manual body fluid cell counting to ensure the highest quality of insights. However, lately there’s been a shift to an automated approach, including analyzer-based body fluid analysis.
How Body Fluids are Analyzed
Body fluid analysis is performed in one of two overall ways: manual cell count using a hemocytometer and an automated approach using an automated analyzer.
While manual counting is the gold standard, there are numerous downsides to the process, as it is time consuming, taking at least 30 minutes to an hour to perform a total RBC and WBC count. Manual review requires a well-trained, licensed personnel due to the testing complexity.
It’s also a subjective approach where competency and technique of testing personnel are not always consistent. Lastly, it’s a labor-intensive process because it requires the staff's full attention and they are unable to perform any other tasks.
After all this manual work, results must be calculated by hand and this can potentially cause clerical and mathematical errors-- even with the use of a calculator.
How Automated Body Fluid Testing is Different
Automated counting removes many of the variables involved with manual review. Foremost, it’s time saving because it takes about five minutes to perform the total RBC and WBC count. It’s moderately complex to perform and can be done by any licensed personnel.
It also reduces the potential for user subjectivity and requires minimal labor. Unlike manual counting, it increases productivity of testing personnel and frees up staff so that they can perform other laboratory tasks.
There are three methods to automated cell counting:
- Method 1: Enhanced Impedance counting is available on some hematology platforms and relies on the displacement of electrical field to provide information on particle size and volume. This aids in classifying particles.
- Method 2: Simple Flow Cytometry measures cell granularity, size and light scatter. This can be performed on some hematology platforms. The laser detects size, volume and shape that aides in classifying particles.
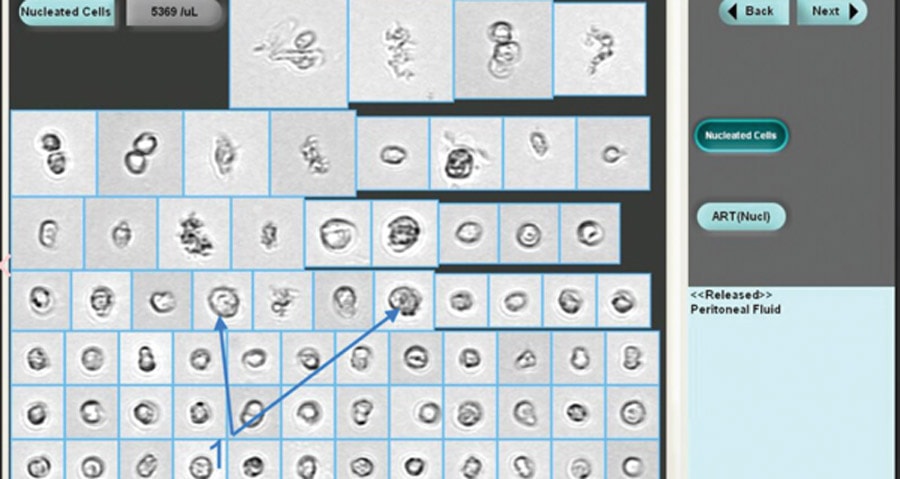
- Method 3: Particle and Image Recognition used by instruments like the iQ200 automated urine microscopy analyzer. The cells are counted by digital flow morphology, allowing the system to capture actual images of particles and classifies by size, shape, contrast and texture. A visual inspection by a technician can be done after analysis is complete, if they need to identify real cells from artifacts.
Do Different Automated Methods Have the Same Functional Sensitivity?
The functional sensitivity for the above-mentioned methods is not the same. For example, in Method #3, the particle and image recognition method offer low count sensitivity and linearity. It can even go down to zero for RBC and WBC.
Also, limitations at the zero of low count range narrows the application of automation for all types of body fluids, particularly clear/colorless CSF. Normally, counting this kind of fluid can only be done by the labor-intensive manual method or by using the iQ200 automated analyzer (Method #3).
However, all the listed automated methods are far less subjective and produce faster results than the gold standard manual method by removing the need for manual counting.
Additionally, when using an automated approach, body fluids with very large cells or cells clumped together like tumor cells cannot be accurately counted by the first two methods. They can be identified by the iQ200 automated analyzer (Method #3) due to the instrument’s ability to identify and capture images of particle during the analysis. iQ200 technicians visually inspect particle images, and when they see very large or clumped tumor cells on the iQ200, the technician is alerted to check test results in hematology for these cells when performing WBC differential counts.

 English
English